The Dawn of Decentralised Nitrate Fertilisers
In the mid-20th century, the Green Revolution significantly transformed the agriculture sector, increasing global food production by nearly 150%. Now, we face the imperative to raise overall food production once again, this time by 70%, to meet the needs of the world’s population, projected to reach about 10 billion by 2050. The challenge is not only to achieve this increase but also to do so in a sustainable and environmentally friendly manner.
In modern food production, synthetic nitrogen (N) fertilisers play a crucial role to the extent that nearly 4 billion people would face starvation without them. However, nitrogen fertilisers come with a substantial environmental cost accounting for about 4% of global greenhouse gas emissions, equivalent to 1300000000000 kg of carbon dioxide equivalent (1.3 Gt CO2eq) for 2021. This amount is approximately half of the total emissions produced in 2021 by the 27 countries of the European Union (EU).
Ammonia is the fundamental building block of nitrogen fertilisers, with the main source of carbon emissions in ammonia production originating from the generation of hydrogen using natural gas feedstock. In response to the call for decarbonization, the industry started shifting towards green hydrogen – hydrogen produced through electrolysis powered by renewable energy.
Is green ammonia the answer to decarbonizing the fertiliser industry? The greenhouse gas (GHG) system boundaries for ammonia synthesis reveal that carbon emissions from the fertiliser use and transport phases (0.84 Gt CO2eq) are nearly twice as high as the emissions generated during manufacturing (0.48 Gt CO2eq). This implies that the industry’s response does not address the root cause of carbon emissions. We need to delve into the fertiliser market to understand why this is the case, as different types of nitrogen fertilisers vary in their carbon emissions during the use phase.

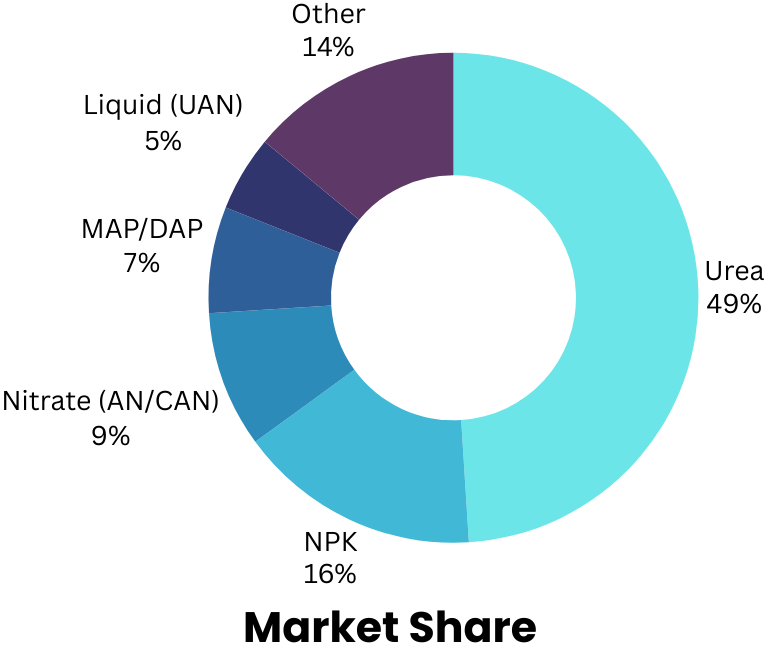
Urea, an ammonia-based fertiliser, is the dominant product in terms of consumption, representing 49% of the total market share. It is produced by combining ammonia with carbon dioxide under high temperature and pressure through a process known as the Bosch-Meiser process. Approximately 1.6 kg carbon dioxide per kg of N is directly captured in the product during the manufacturing phase, to be released when applied in the farm field. Throughout the lifecycle of urea fertiliser, 3.5 kg, 0.1 kg, and 7.0 kg CO2eq are released per kg of nitrogen during the manufacturing (with the best available technique), transportation, and use phases respectively, resulting in a total emission of 10.6 kg CO2eq per kg of N. This implies that the worldwide switch to green ammonia would have a total impact of less than 18%, which pales in comparison to the overall emissions. Thus, considering today’s market structure, we can assert that the potential addressable market for green ammonia in achieving fully decarbonized fertiliser production is relatively small.
Some may argue that, urease inhibitors, when coupled with urea, could reduce emissions during the fertiliser use phase. Simply put, utilizing urease inhibitors is akin to treating the side effects of a medicine with another medication. Not only do urease inhibitors add to the overall cost of fertiliser, but their effectiveness varies depending on soil type, weather conditions, and application methods. Another concern is that prolonged and widespread use of urease inhibitors could lead to the development of resistance in urease-producing microorganisms, diminishing their effectiveness over time.
Would process optimization or the development of a novel catalyst make any difference? Not likely, as only 5% of the total energy is used for nitrogen purification and the catalyst, while 95% is consumed for green hydrogen production by water electrolyzers.
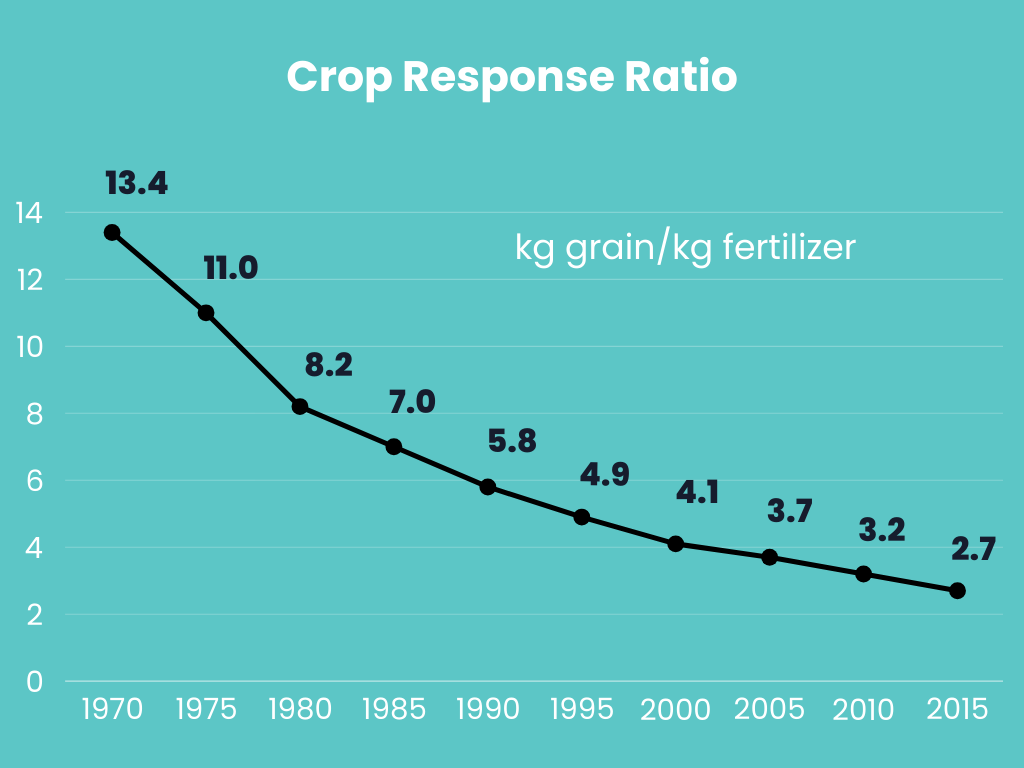
There is more to ammonia-based fertilisers than the emission of greenhouse gases; it also contributes to soil acidification and air pollution. They must undergo conversion to nitrate by soil microorganisms through a process called nitrification. This process acidifies the soil, generating two hydrogen ions for each ammonium molecule nitrified to nitrate. Soil acidification adversely affects soil quality, nutrient availability, and microbial activity, making it a significant factor contributing to soil degradation. The decline in soil productivity is closely tied to soil acification due to overuse of ammonia-based fertilisers. An illustrative example involves Indian farmers who, driven by subsidized prices, have been excessively utilizing urea fertilisers for several decades, resulting in a continuous decline in the crop response ratio—from 13 kg of grains per kg of fertiliser in 1970 to only 2.7 kg of grains per kg of fertiliser in 2015. Considering that 95% of our food comes from soils and 33% of the Earth’s soils are already degraded, the fertiliser industry should be responsible not only for decarbonization but also for maintaining soil health to ensure food security for future generations.
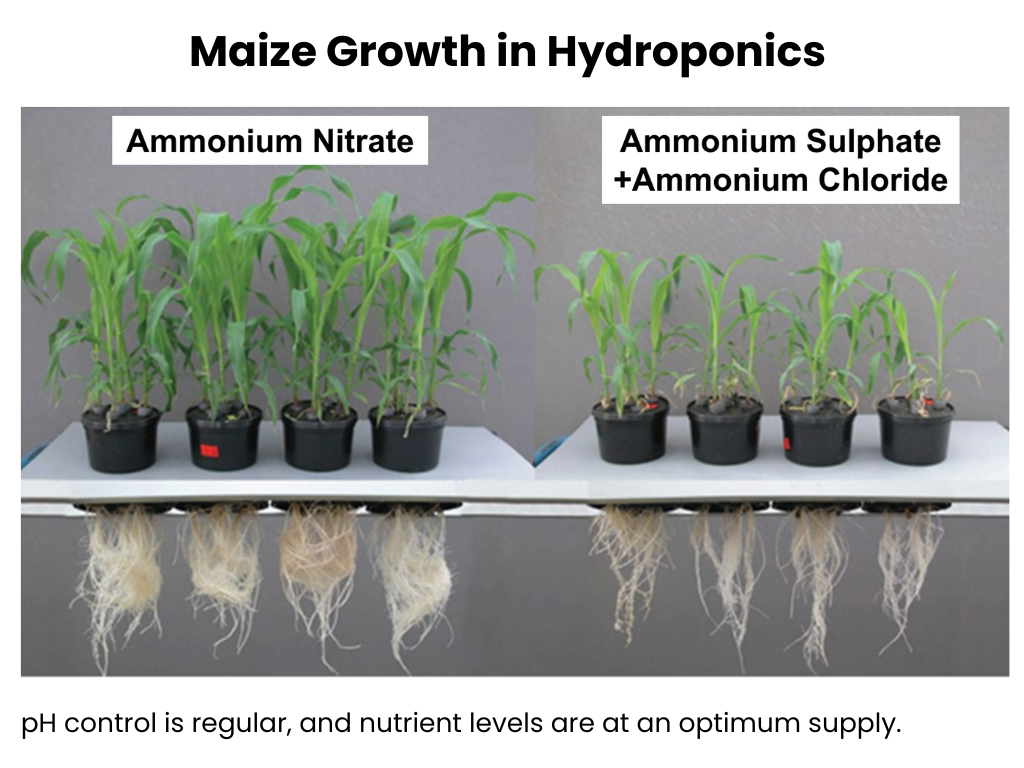
In addition, ammonia-based fertilisers release ammonia gas into the atmosphere through a process called volatilization. Ammonia volatilization represents a significant loss of nitrogen for farmers. In the worst-case scenario, more than 50% of applied nitrogen fertiliser can be lost to the atmosphere (with over 20% being common). Atmospheric ammonia can have a direct impact on respiratory health, and when it reacts with other pollutants in the air, such as sulfuric acid, it forms particulate matter PM2.5, particles with a diameter of 2.5 micrometres or smaller. PM2.5 particles has the ability to penetrate deep into the lungs and cause long-term illnesses, including lung cancer. Approximately 60% of global ammonia emissions result from synthetic nitrogen fertilisers, and in the EU, 50% of PM2.5 air pollution is attributed to atmospheric ammonia.
If overall decarbonization, soil health, and clean air are the objectives, then the holy grail is decarbonised and decentralised nitrate fertiliser production. Nitrate fertilisers offer many advantages over urea fertilisers. They can halve the amount of applied nitrogen fertiliser, providing a more readily available form of nitrogen for plants. Nitrate-based fertilisers can also halve the emissions from the fertiliser use phase compared to the same amount of N from urea fertiliser. This is because nitrate ions, already in the nitrate form, do not undergo the nitrification process in the soil. When the increased nitrogen use efficiency is factored in, nitrate-based fertilisers can effectively reduce emissions from the use phase to one-quarter of that associated with urea fertilisers. Additionally, nitrate ions are not prone to volatilization losses due to their high water solubility. Nitrate-based fertilisers have no acidification potential; in fact, they can reduce soil acidity as plants absorb one hydrogen ion during the uptake of nitrate.
Nitrate-based fertilisers can be produced from green ammonia through an additional multi-step process known as the Ostwald process. This increases complexity and energy requirement of the manufacturing process and thus leads to increasing cost of the final product. Nitrate fertilisers are also subject to more stringent regulatory requirements due to their potential use in the production of explosives. Compliance with safety and security regulations adds to the production costs. This is why urea fertilisers covers the majority of the market today.
An often untold story about green ammonia is the fact that the electrolysis process has a theoretical requirement of about 9 tons of pure or distilled water to produce 1 ton of hydrogen. Taking into account the water purification process, the actual ratio is 20-30 tons of water for every 1 ton of hydrogen. This means that local water stress conditions should be considered in project planning, posing a challenge to scaling up green ammonia and representing a potential threat to local freshwater supply.
It should be clear by now that green ammonia is not the panacea but rather the predicament. We need to change the way we have been fertilizing soil for over a hundred years. Fortunately, nature has provided an elegant solution to this problem: lightning. When lightning strikes water, such an intense energy causes the atmospheric nitrogen molecules to break apart and re-form into nitrogen dioxide molecules. Nitrogen dioxide further reacts with the surrounding water to produce nitrate ions. These nitrate ions are then deposited onto the Earth’s surface through rainfall fertilising the plants. Debye develops a direct nitrogen capture technology based on this principle that allows farmers to produce nitrate fertiliser locally, using only water, air, and electricity.
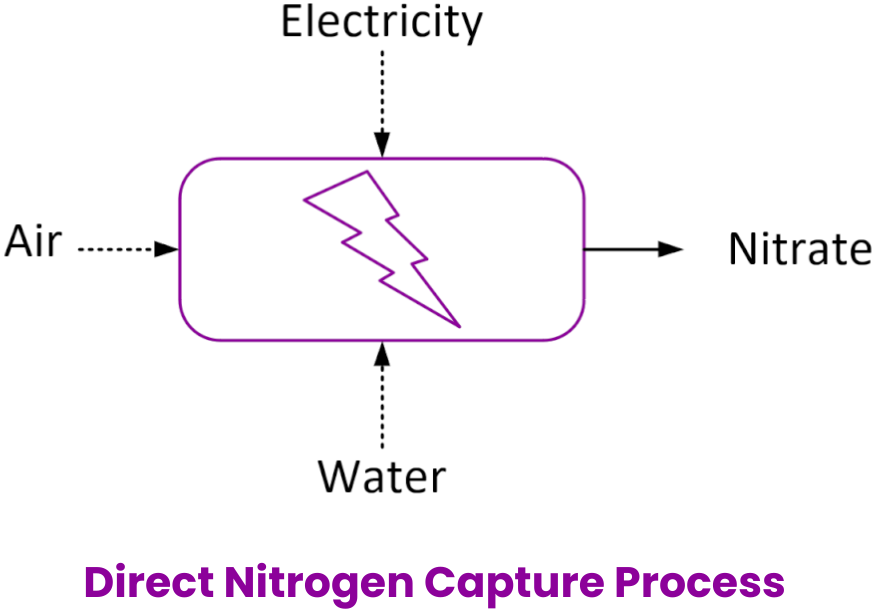
The direct nitrogen capture technology is a single-step, fully electrified process that completely eliminates the need to produce ammonia. This means that hydrogen, and thus associated costly infrastructure, is not required. When powered by renewable energy, it becomes a near zero-emission process, avoiding over 10 kg CO2eq per kg synthetic N during the manufacture, transportation, and fertiliser use phases of urea. Besides, it does not necessitate the distillation of water, as even seawater may be used for the absorption of nitrogen oxide gases. For comparison, 1 metric tonne of Nitrate-N (nitrogen from nitrate-based fertilisers) requires less than 1 metric tonne of water, while 1 metric tonne of green urea-N requires more than 4 metric tonnes of water.
The industrial process to produce green ammonia, known as the Haber-Bosch process, benefits from economies of scale, making it economically viable only for mid-to-large-scale production (> 1000 kg/N per day) due to the substantial upfront capital requirement for infrastructure such as electrolyzers, catalyst reactors, and storage facilities. In contrast, direct nitrogen capture technology can be economically viable at both micro and large scales, ranging from as little as 1 kg N/day to as high as 1000 metric tonnes N/day. This is because the technology is based on a lean process, resulting in the fewest possible components for a plant, thereby enabling highly decentralized and distributed nitrogen fertiliser production. Decentralized production reduces the need for long-distance transportation, leading to cost savings and lower carbon emissions. It is more resilient to disruptions such as natural disasters, supply chain interruptions or geopolitical issues contributing to food stability and security.
At Debye, our mission is to revolutionize the way we produce and use synthetic nitrogen fertilisers through an electrified, decentralized process, with the dual objective of combating climate change, and enhancing food stability.
We believe that true progress comes from reimagining the status quo entirely, not merely refining existing practices. As Oren Harari put it, the electric light did not come from the continuous improvement of candles.
We invite you to follow us on this exciting journey as we disrupt the fertiliser industry to build a zero-carbon future!










